EDUCATION/PROFESSIONAL BACKGROUND
Dr. Yakubenko received a Ph.D. in biochemistry from the National Academy of Sciences of Ukraine. He obtained a post-doctoral training in cell and molecular biology at the Lerner Research Institute at the Cleveland Clinic. He was a research faculty in the Cleveland Clinic before joining ETSU in 2015.
RESEARCH INTERESTS
My laboratory focuses on elucidating the mechanisms of leukocyte migration during acute and chronic inflammation.
Leukocyte adhesive receptors, integrins αDβ2 and αMβ2.
A major area of interest is the family of cell-surface receptors, integrins, which mediate interactions with extracellular ligands and transduce signals to the cytoskeleton, thereby regulating cell adhesion, migration, and intracellular signaling under both physiological and pathological conditions. In particular, we study the roles of the β2 integrins - αDβ2 and αMβ2 in macrophages and neutrophils during the development of acute inflammatory responses, such as sepsis, and chronic inflammatory diseases, including atherosclerosis.
End-products of polyunsaturated fatty acids oxidation – carboxyalkylpyrroles (CEP, CPP)
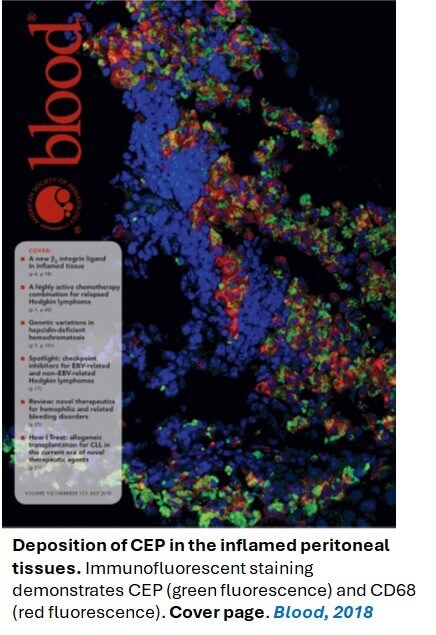
Recently, we have focused on the pathophysiological effects of the product of the oxidation of polyunsaturated fatty acids (PUFAs) (e.g. γ-linolenic, arachidonic, and docosahexaenoic (DHA)) on the macrophage and neutrophil recruitment and accumulation during inflammation. It has been shown that PUFA peroxidation in the inflamed tissue generates the formation of the end products, carboxyalkylpyrroles (CAPs), which form covalent adducts with proteins via the ε-amino group of lysines. CAPs formation in the tissue is dramatically increased in chronic inflammation, particularly during atherosclerosis. We recently found that a product of DHA oxidation, carboxyethylpyrrole (CEP) and product of arachidonic acid oxidation, carboxypropylpyrrole (CPP) can interact with integrins αDβ2 and αMβ2. CAP-modified ECM serves as an adhesive substrate and regulates an integrin β2-mediated migration of macrophages and neutrophils to the site of inflammation. The goal of this project is to define how CAP-mediated modification of the extracellular matrix regulates leukocyte migration in different inflammatory diseases.
α7 nicotinic acetylcholine receptor (α7nAChR)
Another major project in our laboratory focuses on the contribution of the α7 nicotinic acetylcholine receptor (α7nAChR) to neutrophil and macrophage migration during inflammation. α7nAChR is a ligand-gated ion channel that, in addition to its classical neuronal expression, is also expressed by several immune cell types, including lymphocytes, neutrophils, and macrophages. It has been well established that stimulation of the vagus nerve—the primary parasympathetic pathway—activates macrophage-expressed α7nAChR, thereby engaging the cholinergic anti-inflammatory pathway. Activation of α7nAChR on macrophages triggers downstream intracellular signaling cascades, including suppression of NF-κB activation, leading to reduced production of TNF-α and other pro-inflammatory cytokines.
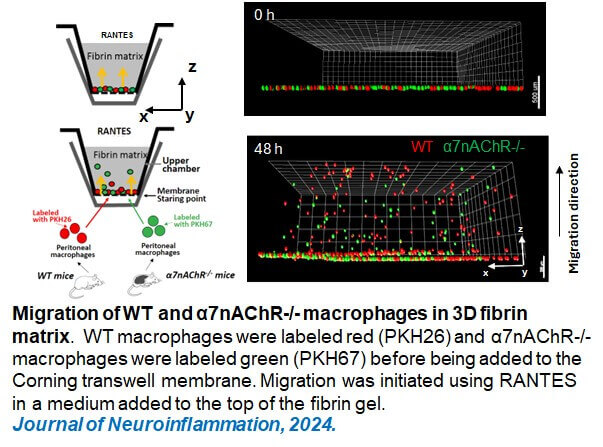
In this project, we discovered that α7nAChR signaling also plays a critical role in regulating macrophage migration. Using complementary in vitro and in vivo approaches, we demonstrated that α7nAChR deficiency significantly impairs macrophage migration within three-dimensional matrices and during endotoxemia-induced recruitment to inflamed lungs, resulting in increased mortality in mice. Our recent findings further indicate that α7nAChR activation modulates leukocyte migration through mechanisms involving β2 integrins.
The overall goal of this project is to elucidate the molecular mechanisms by which the autonomic nervous system regulates neutrophil and macrophage migration through α7nAChR signaling on immune cells.
We used a range of protein chemistry, cell biology, and molecular biology approaches in our studies, including molecular cloning and mutagenesis; expression and analysis of recombinant proteins; adhesion and migration assays of primary leukocytes and transfected cells; real-time PCR; fluorescence microscopy; flow cytometry; imaging flow cytometry; and mouse models of inflammation, including atherosclerosis, diabetes, endotoxemia, and peritonitis.
RESEARCH SUPPORT
Ongoing:
National Institute of Health, NHLBI
R15 HL157836-01 Yakubenko (PI: Yakubenko), 02/01/2022-01/31/2026
“The role of a neuro-immune synapse in macrophage migration”.
The major goal of this award is to evaluate the role of α7nAChR in macrophage migration during the inflammatory response.
Completed at ETSU:
American Heart Association
20AIREA35150018 (PI: Yakubenko), 01/01/2020-12/31/2022
“Targeting the product of DHA oxidation as potential substrate for macrophage retention during atherosclerosis”.
The major goal of this study is to determine the molecular mechanism of interaction between macrophage integrins and ECM proteins modified by the end-product of DHA oxidation during the development of atherosclerosis.
National Institute of Health, NIDDK
1R01 DK102020-01 (PI: Yakubenko), 07/01/2014 - 04/30/2020
“Role of β2 integrins in macrophage retention and egress during inflammation”.
The purpose of this proposal is to test the role of leukocyte migratory receptors, integrins αMβ2 and αDβ2, in the retention and egress of macrophages within the site of chronic inflammation during the development of metabolic syndrome.
American Heart Association, Great Rivers Affiliate, Grant-in-Aid
14GRNT20410074 (PI: Yakubenko), 07/01/2014 – 06/30/2016
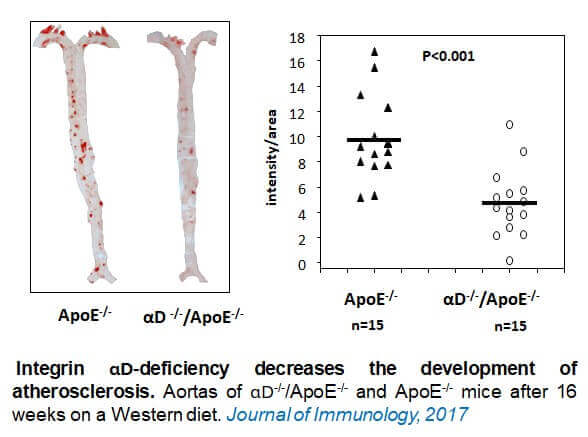
“The contribution of integrin αDβ2 to the macrophage migration and lipid deposition during atherogenesis.”
This project will focus on the determination of the impact of integrin αDβ2 on the development of atherosclerosis, focusing on both components of early atherogenesis – macrophage accumulation and lipid deposition.
PUBMED
Complete List of PubMed Publications
SELECTED RECENT PUBLICATIONS (from 46 total)
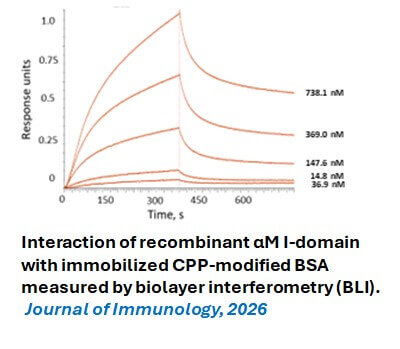
- Casteel JL, Phillips T, Hopke A, Ricker S, Gao D, Podrez EA, Byzova TV, Yakubenko VP. Product of Arachidonic Acid Oxidation Inhibits Neutrophil Migration by Forming the Inflammatory Substrate for Integrin αMβ2. Journal of Immunology (Accepted Dec 1, 2025).
- Keever KR, Kui Cui, Casteel JL, Singh S, Hoover DB, Williams DL, Pavlov VA, Yakubenko VP. Cholinergic signaling via the α7 nicotinic acetylcholine receptor regulates the migration of monocyte-derived macrophages during acute inflammation. Journal of Neuroinflammation, 2024 Jan 4;21(1):3. PMID: 38178134.
- Casteel JL, Keever KR, Ardell CL, Williams DL, Gao D, Podrez EA, Byzova TV, Yakubenko VP. Modification of extracellular matrix by the product of DHA oxidation switches macrophage adhesion patterns and promotes retention of macrophages during chronic inflammation. Frontiers in immunology. 2022 May 26;13:867082.
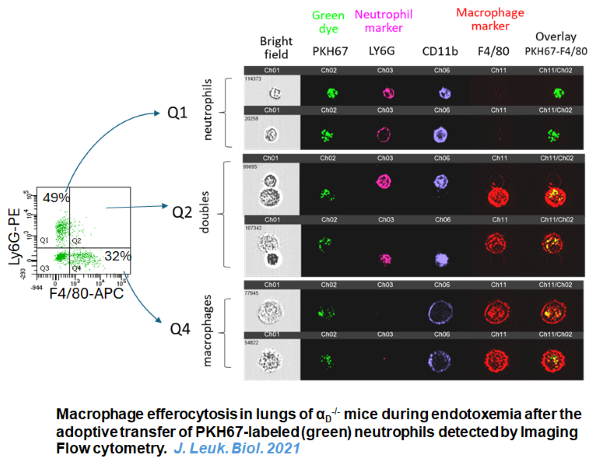
- Bailey WP, Cui K, Ardell CL, Keever KR, Singh S, Rodriguez-Gil DJ, Ozment TR, Williams DL, Yakubenko VP. The expression of integrin αDβ2 (CD11d/CD18) on neutrophils orchestrates the defense mechanism against endotoxemia and sepsis. J Leuk Biol. 2021 May;109(5):877-890. The paper was selected as Frontline Science and received Editorial commentary: Liwu Li. J Leuk Biol. 2021 109(5):861-863.
- Cui K, Podolnikova NP, Bailey W, Szmuc E, Podrez EA, Byzova TV, Yakubenko VP. Inhibition of Integrin αDβ2-mediated Macrophage Adhesion to End-product of DHA Oxidation Prevents Macrophage Accumulation during Inflammation. J Biol Chem. 2019 Sep 27;294(39).
- Cui K, Aziz M, Ardell CL, Podolnikova NP, Yakubenko VP. Distinct migratory properties of resident, classically and alternatively-activated macrophages are regulated by αDβ2 and αMβ2 integrin-mediated adhesion. Frontiers in immunology. 2018; 9:2650.
- Yakubenko VP, Cui K, Ardell CL, Brown KE, West XZ, Salomon RG, Podrez EA, Byzova TV. Oxidative modifications of extracellular matrix promote the second wave of inflammation via β2 Integrins. Blood. 2018 Jul 5;132(1):78-88.
- Wolf D., Anto-Michel N., Blankenbach H., Wiedemann A., Buscher K., Hohmann JD, Lim
B, Bäuml M, Marki A, Mauler M, Duerschmied D, Fan Z., Winkels H, Sidler D, Diehl P,
Zajonc D., Hilgendorf I., Stachon P, Schell M, Sommer B., von Muhlen K., Plow E.,
Yakubenko V, Libby P., Bode C., Ley K., Peter K., and Zirlik A. A ligand-
specific blockade of the integrin Mac-1 selectively targets pathologic inflammation while maintaining protective host-defense. Nature Communications 2018 Feb 6;9(1):525. - Aziz M, Cui K, Das M, Brown KE, Ardell CL, Febbraio M, Pluskota E, Wu H, Ballantyne CM, Smith JD, Cathcart MK, Yakubenko VP. The upregulation of integrin αDβ2 (CD11d/CD18) on inflammatory macrophages promotes macrophage retention in vascular lesions and development of atherosclerosis. Journal of Immunology. 2017 Jun 15;198(12):4855-4867.
- Yakubenko VP, Byzova TV. Biological and pathophysiological roles of end-products of DHA oxidation. Biochim. Biophys. Acta. 2017 Apr;1862(4):407-415.
- Liu J, Das M, Yang J, Ithychanda SS, Yakubenko VP, Plow EF, and Qin J. Structural mechanism of integrin inactivation by filamin. Nature Structural and Molecular Biology 2015 May;22(5):383-9.
 Stout Drive Road Closure
Stout Drive Road Closure